Abstract
Introduction: Clones characterized by gene mutations independent of BCR::ABL1 have been identified in approximately 20-30% of newly diagnosed patients with chronic myeloid leukemia (CML) in chronic phase, whereby mutations in epigenetic modifier genes were most common. These findings prompted the systematic analysis of prevalence, dynamics, and prognostic significance of such mutations in a prospective clinical trial.
Methods: 222 CML patients from the ongoing German TIGER study (CML-V; NCT01657604) were investigated at diagnosis by targeted next-generation sequencing covering 54 myeloid leukemia-associated genes. Median age of patients was 52 years (range, 18-79 years), 140 (63%) were male. All patients received nilotinib based tyrosine kinase inhibitor therapy. To study mutation dynamics, longitudinal deep sequencing analysis of serial samples was performed for 100 patients after 12, 24, and 36 months of therapy.
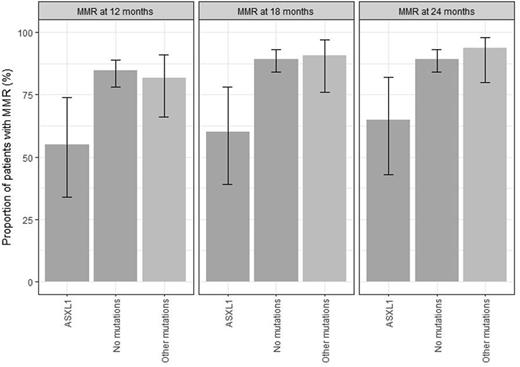
Results: At diagnosis, 53/222 CML patients (24%) carried 60 mutations in addition to BCR::ABL1 affecting the genes ASXL1, ATRX, BCOR, BCORL1, CALR, CBL, CBLB, CUX1, DNMT3A, FBXW7, GATA2, GNAS, IKZF1, JAK2, KDM6A, PHF6, RUNX1, SMC3, TET2, U2AF1, and WT1. ASXL1 mutations were most frequently identified and were observed in 20 patients (9%). For the predictive analysis, the ASXL1 c.1934dup mutation was excluded based on frequent sequencing artifacts in this homopolymer area. Six patients showed mutations in more than one gene. Median age of patients carrying additional mutations was 54 years (range, 19-78 years). Follow up analysis of 100 patients revealed three distinct mutation patterns under therapy, including eradication in parallel to a decline of BCR::ABL1 transcripts (mutations on top of BCR::ABL1, 19 patients), persistence despite decline of BCR::ABL1 transcripts (BCR::ABL1 on top of a preexisting mutation, 5 patients), and emergence (clonal evolution during therapy, 13 patients) of novel mutations within ASXL1, BCOR, DNMT3A, IKZF1, JAK2, STAG2, TET2, and TP53. Mutation dynamics in six patients with multiple mutations showed parallel patterns of mutant clones in two and non-parallel patterns in four patients. Concerning clinical impact of mutations, patients carrying an ASXL1 mutation at diagnosis showed a less favorable molecular response to nilotinib treatment, as a major molecular response (MMR) was achieved less frequently at month 12 (55% vs. 85%; p=0.0036), month 18 (60% vs. 89%; p=0.0018), and month 24 (65% vs. 89%; p=0.0076) compared to patients without mutations. Investigating the response categories MR4, MR4.5, and MR5, no statistically significant and clinically relevant differences between "no mutations" and either "ASXL1" or "other mutations" were found. Patients with ASXL1 mutation were more frequently found in the high risk category according to the EUTOS score (37%) compared to low risk patients (6%, p=0.0004). Moreover, patients with ASXL1 mutations were significantly younger than patients with other mutations (median 47 vs. 56 years, p=0.0380).
Conclusions: In newly diagnosed CML patients in chronic phase, mutations in addition to BCR::ABL1 were frequently identified at diagnosis and were found to vary in their dynamics during treatment. ASXL1 mutations were most common and affected patients showed MMR less frequently at month 12, 18, and 24 compared to all other patients. ASXL1 may, after further validation, serve as an additional prognostic factor for molecular response in newly diagnosed CML patients.
Disclosures
Ernst:Janssen: Other: travel grant. Brümmendorf:Pfizer: Consultancy, Honoraria, Other: travel support, Research Funding; Merck: Consultancy, Other: travel support; Janssen: Consultancy, Other: travel support; Novartis: Consultancy, Honoraria, Other: travel grant, Research Funding. Burchert:Incyte: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; AOP Health: Honoraria, Research Funding. le Coutre:Novartis: Honoraria; Incyte: Honoraria; Pfizer: Honoraria. Krause:Art-tempi: Honoraria; Kosmas: Honoraria; Abbvie: Other: Expenses. Saussele:Novartis: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Roche: Honoraria; Pfizer: Honoraria. Hochhaus:BMS: Research Funding; Pfizer: Research Funding; Incyte: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal